Point-of-Care Tests: Hepatitis C
A range of Point-of-Care tests are available to diagnose hepatitis C, including antibody and RNA tests.
Click the headings below to find out more. ecstt is. 2018;217:1889-96.
.
Dried blood spot (DBS) tests are ideal for simple testing of people in a high-risk group for hepatitis C. These tests are very easy to collect, allowing them to be completed at home or by community and healthcare workers. Dried blood spot testing provides a low threshold testing option and reduces the need for venepuncture.
In Australia, these tests are not yet widely used outside of NSW, which is conducting a DBS research study. People who are eligible for this study can register online to have test kit mailed out to them. For information on who is eligible, please visit: https://www.dbstest.health.nsw.gov.au/The-Test

How it works
- The finger is pricked using a lancet to get a blood drop.
- The blood drop is applied to filter paper, which is marked to indicate where to place samples.
- When the blood has dried on the filter paper, the kit is sent to a laboratory for testing. The test kits provide information and equipment to ensure that a suitable sample is obtained and that it is not contaminated during transport.
- Some labs will test for HCV antibodies and only proceed to RNA testing if antibodies are present. Other labs will go straight to HCV RNA testing. This will often differ by region and target population, according to HCV prevalence.
- Results may be returned to the person’s general practitioner, or another healthcare professional will call the person to deliver their results and connect them with the relevant care. If HCV antibodies are present, the person will need to be tested for HCV RNA, indicating the presence of active infection.
Samples are stable at room temperature for around two weeks if stored correctly. HIV and other viruses can also be tested using the same method.
Further information
Explore the resources below to learn more about how DBS testing works and the considerations when using this intervention in your service.
- NSW Health DBS Test (website)
- How to collect a DBS sample - INHSU (1 page doc)
- How to collect blood spot sample (animated patient video)
- Collecting a valid DBS sample - INHSU (1 page doc)
- DBS collection and HCV virus testing - The Kirby Institute (step-by-step guided video)
- DBS result interpretation -INHSU (2 page doc)
- INHSU’s Dried Blood Spot (DBS) Testing for Hepatitis C How-To Guide
Evidence
- Cunningham, E. B., Wheeler, A., Hajarizadeh, B., French, C. E., Roche, R., Marshall, A. D., Fontaine, G., Conway, A., Valencia, B. M., Bajis, S., Presseau, J., Ward, J. W., Degenhardt, L., Dore, G. J., Hickman, M., Vickerman, P., & Grebely, J. (2022). Interventions to enhance testing, linkage to care, and treatment initiation for hepatitis C virus infection: a systematic review and meta-analysis. The Lancet Gastroenterology & Hepatology, 7(5), 426–445. https://doi.org/10.1016/s2468-1253(21)00471-
Lateral flow tests provide a near-instant blood test result indicating the presence of HCV antibodies in a drop of blood. In Australia, these tests are currently being reviewed by the Therapeutic Goods Administration (TGA), and are likely to receive approval in the near future.
Once approved, lateral flow tests will enable healthcare practitioners in Australia to quickly identify whether a person should have additional testing for HCV RNA. Results are usually clear in less than 20 minutes.

How it works
- The finger is pricked using a lancet to get a blood drop, which is collected in a capillary tube.
- The sample is dispensed into the sample well (marked with an S). An assay diluent is also added to the specimen well.
- After 5-20 minutes, one or two lines will appear in the window, indicating whether antibodies are present.
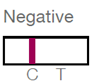
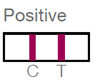
A positive result indicates that the person needs HCV RNA testing to check for active infection.
While lateral flow tests are largely reliable, they may give false positives or false negatives. If HCV is suspected and the person receives a negative result from a lateral flow test, further testing may be warranted.
Further information
- Currently the Abbott Bioline™ HCV assay is being reviewed for use in Australia by the TGA.
- Abbott Bioline™ factsheet (refer to page 18)
Evidence
- Cunningham, E. B., Wheeler, A., Hajarizadeh, B., French, C. E., Roche, R., Marshall, A. D., Fontaine, G., Conway, A., Valencia, B. M., Bajis, S., Presseau, J., Ward, J. W., Degenhardt, L., Dore, G. J., Hickman, M., Vickerman, P., & Grebely, J. (2022). Interventions to enhance testing, linkage to care, and treatment initiation for hepatitis C virus infection: a systematic review and meta-analysis. The Lancet Gastroenterology & Hepatology, 7(5), 426–445. https://doi.org/10.1016/s2468-1253(21)00471-4
The Xpert® HCV viral fingerstick assay uses a small sample of blood to test for HCV RNA, with results being returned in around 60 minutes. In this testing method, the sample is collected into a special cartridge that is loaded into the GeneXpert System. Whereas many other POCT methods require a follow-up test for HCV RNA, this approach delivers an RNA result in one test. The healthcare professional can then offer treatment as soon as this result is returned.
How it works
- The finger is pricked using a lancet to get a blood drop, which is collected in a capillary tube.
- The sample is dispensed into a Minivette® cartridge, which is loaded into the GeneXpert System.
- Results are returned within 60 minutes.
Further information
- Journey Inside the Cepheid GeneXpert® Cartridge – (3D Animation)
- Point-of-care hepatitis C virus RNA testing - The Kirby Institute (A step-by-step guided video)
- Xpert® HCV VL Fingerstick Datasheet (PDF)
- Simplification of testing and treatment in the quest to eliminate hepatitis C infection (Webcast)
- GeneXpert Dx System (Operator Manual)
- GeneXPert Maintenance Guide
- Cepheid Xpert TB Testing Outline
- Quick start guide: Xpert HCV VL FS
- Assay Technical Training Xpert HCV VL Fingerstick
- INHSU’s Point-of-Care RNA Testing for Hepatitis C How-To Guide
Evidence
- Cunningham, E. B., Wheeler, A., Hajarizadeh, B., French, C. E., Roche, R., Marshall, A. D., Fontaine, G., Conway, A., Valencia, B. M., Bajis, S., Presseau, J., Ward, J. W., Degenhardt, L., Dore, G. J., Hickman, M., Vickerman, P., & Grebely, J. (2022). Interventions to enhance testing, linkage to care, and treatment initiation for hepatitis C virus infection: a systematic review and meta-analysis. The Lancet Gastroenterology & Hepatology, 7(5), 426–445. https://doi.org/10.1016/s2468-1253(21)00471-4
